SCIENCE-BACKED BEAUTY: Redefining Trust Through Clinical Testing

The beauty industry stands at a turning point where science, safety, and transparency define success. As consumers demand proof over promises, “clinically tested” has evolved from a marketing phrase into the benchmark of credibility. Once reserved for premium brands, clinical validation is now the gold standard for proving real results – especially as concerns over “forever chemicals” and hidden contaminants grow worldwide.
Clinical Testing: Turning Promises into Proof
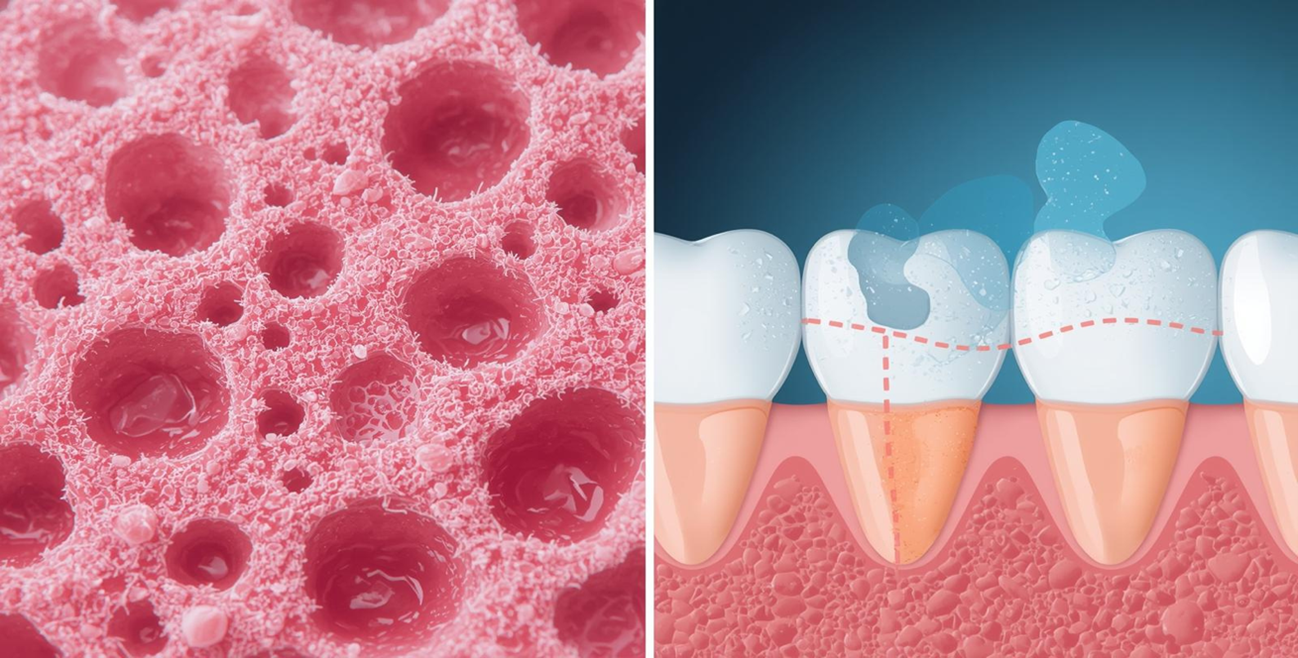
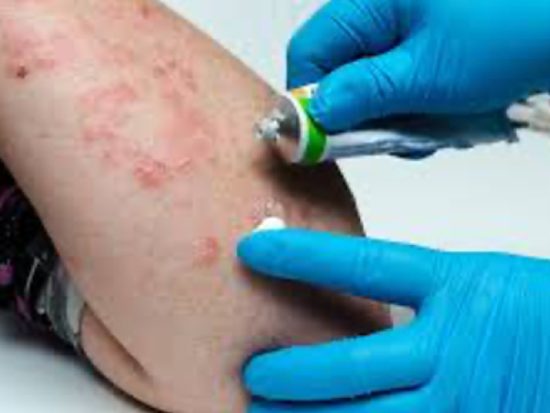
Clinical testing goes beyond perception – it’s about evidence-based results. Through controlled studies on real people and advanced dermatological imaging, researchers measure changes in hydration, firmness, and wrinkle depth with precision. Unlike subjective claims, brands can now state quantifiable outcomes like “reduces fine lines by 25%.” Though more demanding, this process builds authentic trust among ingredient-conscious consumers and safeguards against misleading “clinically proven” claims.
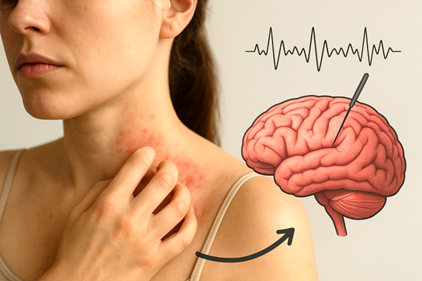
Neuroimaging studies show that itch is processed by a complex itch matrix in the brain, involving sensory, motor, and emotional regions, much like pain. In AD patients, the basal ganglia, which controls movement and habit formation, is more active, while healthy individuals show stronger responses in regions like the thalamus and insula. This suggests that in AD, brain circuits tied to planning and repetitive behaviours—the corticostriatal pathway—are heavily involved, making scratching deeply wired rather than just reflexive. Understanding these brain-skin connections opens new possibilities for therapies that target not just the skin, but also the neurological drivers of itch.
Safety First: Because Results Mean Nothing Without Trust
True beauty is safe beauty. Every formulation must undergo rigorous safety evaluation – testing for irritation, microbial contamination, heavy metals, and PFAS — to ensure it’s both gentle and stable. Verified “PFAS-free,” “dermatologist-tested,” and “non-irritating” claims reassure consumers while reinforcing brand credibility. When efficacy meets safety, beauty transcends appearance and becomes a relationship built on trust.
The Future: Science-Backed Integrity
With the cosmetic testing market projected to double from USD 0.53 billion in 2024 to 1.11 billion by 2033, innovation is accelerating through AI-driven analytics, in vitro methods, and precision imaging. India’s growing formulation landscape is emerging as a global hub for validated, science-backed beauty.
NovoBliss Research: Where Beauty Meets Evidence
At NovoBliss Research, we drive integrity-driven innovation – merging clinical science, safety validation, and advanced efficacy assessment to generate quantifiable evidence behind every product claim.
Our mission: To advance integrity in beauty by converting every claim into quantifiable proof and every product into a trusted scientific outcome