Pharmaceutical Clinical Research

NovoBliss Research provides pharmaceutical testing solutions designed to ensure uncompromising safety, quality, and regulatory compliance. Operating to the highest global standards, including CDSCO, US FDA, and EMA | COLIPA guidelines, our approach integrates scientific precision with rigorous quality systems to support reliable product development. Through validated methodologies, advanced technologies, and collaborative expertise, we generate robust data that strengthens regulatory submissions, enhances product credibility, and builds global trust.
-
PK Endpoints Studies
Design and conduct studies to assess the absorption, distribution, metabolism, and excretion of compounds, providing critical data for dose optimization and regulatory submissions.
-
Bioavailability Studies
Evaluate the rate and extent of absorption of the active pharmaceutical ingredient (API) into systemic circulation, serving as a critical tool in formulation development, dosage form optimization, and regulatory approval.
-
AUC (Area Under the Curve)
-
Cmax (Maximum Concentration)
-
Tmax (Time to Reach Cmax)
-
Kel (Elimination Rate Constant)
-
Relative Bioavailability (%)
-
t½ (Elimination Half-life)
-
Phase – I to IV
Manage and execute clinical trials across all phases, from first-in-human studies to post-marketing surveillance, ensuring adherence to regulatory standards and scientific integrity.
-
Post Marketing Observational | RWE Studies
Collect and analyze real-world data to assess product performance, safety, and patient outcomes in diverse populations, supporting long-term product strategies.
-
Investigator Initiated Studies | Trials
Support investigator-led research initiatives, providing expertise in study design, regulatory compliance, and data analysis to advance scientific knowledge.
-
Clinical Trial Management
Offer end-to-end management of clinical trials, including protocol development, site selection, monitoring, data management, and regulatory reporting, ensuring efficient and compliant study execution.
-
Remote Monitoring
Implement technology-driven solutions to facilitate remote patient monitoring and decentralized clinical trial operations, enhancing efficiency and patient-centricity.
Assessment:
Pharmaceutical Research Expertise
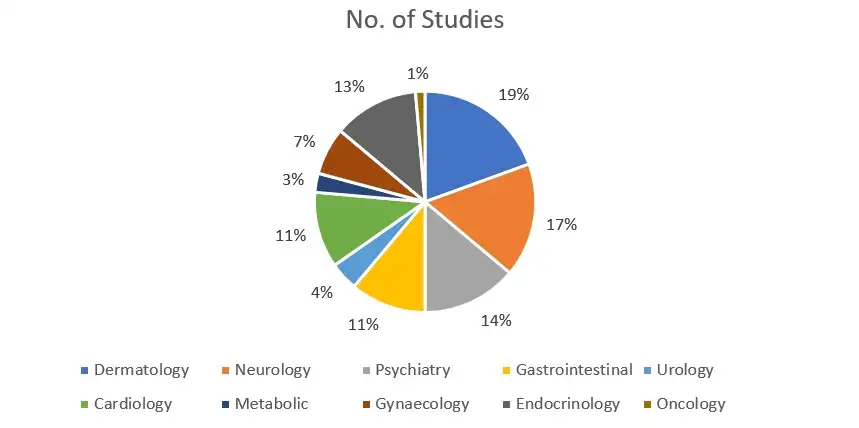
At NovoBliss Research, we have executed clinical studies across multiple therapeutic domains including Dermatology, Neurology, Psychiatry, Gastrointestinal, Urology, Cardiology, Metabolic Disorders, Gynaecology, Endocrinology, and Oncology. Our clinical trials are primarily driven by structured data-collection and evidence-generation initiatives. With proven experience in over 14 therapeutic areas, we offer scientifically rigorous, protocol-compliant, and regulatory-aligned research solutions that support high-quality clinical outcomes.