Regulatory Aspects of Cosmetic Testing
 Cosmetic Legislation frameworks in the major markets are based on either broad or narrow definitions of cosmetics. A legal framework with broad definitions of cosmetic work includes extensive restriction lists of ingredients whereas one with a narrow definition does not feature restriction lists of ingredients but may classify a product as a drug on the basis of claims made. In general, the regulatory basics for cosmetics are different for different countries. Even the classification rules defining what a cosmetic is differed between the countries. Some countries may classify a product as cosmetic whereas other countries classify the same as a drug. This has implications for the testing of cosmetics. Depending on which country the testing is performed and/or the test product is to be marketed, different specific testing may be required, desired and/or allowed.
We are fortunate enough to find some universal similarities in the world of the differences in regulatory basics for cosmetics in different countries like-
Cosmetic Legislation frameworks in the major markets are based on either broad or narrow definitions of cosmetics. A legal framework with broad definitions of cosmetic work includes extensive restriction lists of ingredients whereas one with a narrow definition does not feature restriction lists of ingredients but may classify a product as a drug on the basis of claims made. In general, the regulatory basics for cosmetics are different for different countries. Even the classification rules defining what a cosmetic is differed between the countries. Some countries may classify a product as cosmetic whereas other countries classify the same as a drug. This has implications for the testing of cosmetics. Depending on which country the testing is performed and/or the test product is to be marketed, different specific testing may be required, desired and/or allowed.
We are fortunate enough to find some universal similarities in the world of the differences in regulatory basics for cosmetics in different countries like-
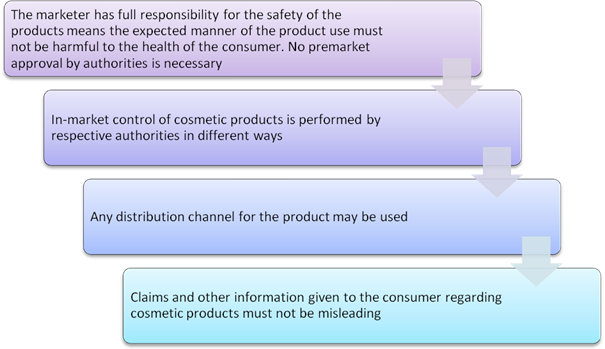 As discussed in the beginning, the cosmetic regulation framework i.e. broad framework and narrow framework. The broad framework roughly describes the European Union (EU) cosmetics regulation where they have employed extensive lists with restrictions for specific ingredients as well as positive lists for allowed ingredients and require safety data to be available. It is not surprising that many countries like ASEAN countries, Mercosur countries, the Andean Pact countries as well as South Africa have similar cosmetic regulations as it is successful in regulating cosmetic product safety as well as allowing innovation of cosmetic products.
India is also a large market that was of less interest to major cosmetic manufacturers in the past, probably because of the low average income of the population. Considering its economic growth, it has become increasingly important to have cosmetic regulation. So, India developed its cosmetic regulation which is completely integrated with its drug regulatory system. Cosmetic guidelines are governed by the Drugs and Cosmetic Act, and the official government body is the CDSCO (Central Drugs Standard Control Organization) headed by DCGI (Drug Controller General of India).
To get marketing approval for cosmetics, the products must comply and be coherent with BIS (Bureau of Indian standards) guidelines which are put forward by CDSCO to ensure the safety of consumers. Certain documents like COAs (Certificate of Analysis), INCIs (International Nomenclature Cosmetic Ingredients), heavy metal, non-animal declaration, testing methods, labels with a description of product n direction for usage, manufacturing license, import registration in case of imports should be according to BIS guidelines and are required by regulatory approval for marketing of cosmetic products.
The narrow framework model roughly defines the cosmetic regulatory system in the USA. It has been discussed by O.Wunderlich that India too follows the same framework similar to the USA. Let’s see the difference between the requirements of India and the USA in the following table:
As discussed in the beginning, the cosmetic regulation framework i.e. broad framework and narrow framework. The broad framework roughly describes the European Union (EU) cosmetics regulation where they have employed extensive lists with restrictions for specific ingredients as well as positive lists for allowed ingredients and require safety data to be available. It is not surprising that many countries like ASEAN countries, Mercosur countries, the Andean Pact countries as well as South Africa have similar cosmetic regulations as it is successful in regulating cosmetic product safety as well as allowing innovation of cosmetic products.
India is also a large market that was of less interest to major cosmetic manufacturers in the past, probably because of the low average income of the population. Considering its economic growth, it has become increasingly important to have cosmetic regulation. So, India developed its cosmetic regulation which is completely integrated with its drug regulatory system. Cosmetic guidelines are governed by the Drugs and Cosmetic Act, and the official government body is the CDSCO (Central Drugs Standard Control Organization) headed by DCGI (Drug Controller General of India).
To get marketing approval for cosmetics, the products must comply and be coherent with BIS (Bureau of Indian standards) guidelines which are put forward by CDSCO to ensure the safety of consumers. Certain documents like COAs (Certificate of Analysis), INCIs (International Nomenclature Cosmetic Ingredients), heavy metal, non-animal declaration, testing methods, labels with a description of product n direction for usage, manufacturing license, import registration in case of imports should be according to BIS guidelines and are required by regulatory approval for marketing of cosmetic products.
The narrow framework model roughly defines the cosmetic regulatory system in the USA. It has been discussed by O.Wunderlich that India too follows the same framework similar to the USA. Let’s see the difference between the requirements of India and the USA in the following table:
| Regulation | USA | India |
| Agency | FDA | CDSCO |
| Regulation | Food, Drug and Cosmetic Act | Drugs and Cosmetic Act |
| Pre-Market Approval | Not Required | Required from state authorities |
| Safety | Responsibility of manufacturer | Manufacturer is asked to maintain records and furnish valid COAs and INCIs. |
| Labelling Declaration | FDA 21 CFR 701 & 740 | According to BIS, FSSAI, AYUSH as applicable |
| Label Language | English | English |
